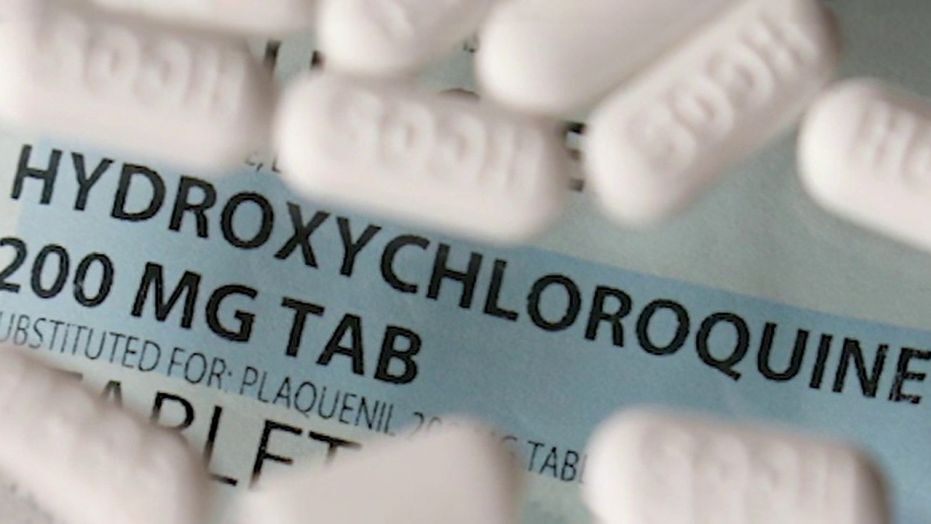
On June 4, 2020, three of this study the authors have decided to withdraw the study. For questions about collected data from Surgisphere, data collection organization has launched an independent review authors; Surgisphere not provide complete information to the independent expert and the test can not be performed, leading the authors to withdraw the paper. so far in the largest observational study, hydroxychloroquine drug as a treatment for COVID-19 to investigate, the researchers found little evidence that it helps, and worrying signs that the drug can cause damage. In a study published May 22 in the journal Lancet, scientists in the United States and Switzerland report on an analysis of more than 96,000 people with confirmed COVID-19 for the hospital of 671 hospitals across six continents. Almost 15,000 patients were treated with one of the following: chloroquine (which is an older version of hydroxychloroquine), hydroxychloroquine, or one of these two drugs in combination with an antibiotic. The rest received none of these medications and served as controls. People in one of four treatment groups were more likely during their hospitalization than those who do not die untreated. People either chloroquine or hydroxychloroquine received were only 16% to 18% more often in the hospital dying is not receiving the drug compared to those, and they were with these drugs in combination with an antibiotic 22% to 24% treated rather die in the hospital. These risks are also remained significant after the researchers to control factors like smoking, underlying heart disease, diabetes, lung disease or immune system diseases. It also had those who received higher risk of cardiac arrhythmia to develop drugs – a known risk factor for chloroquine and hydroxychloroquine – than people who were not treated with medication. “But we sliced and diced the data, the results were identical,” says Dr. Mandeep Mehra, chairman of cardiovascular medicine at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School who led the study. “There was no evidence of benefit, and a coherent signal of damage -. And, in particular, damage to cardiac arrhythmia connected” The results come as claimed by the US President Donald Trump taking hydroxychloroquine, the doctor asked the House white, because “heard many good stories.” Mehra notes that the study only people with diagnosed COVID-19 included, while the president has not been reported to be infected. Hydroxychloroquine is approved for the treatment of malaria – to replace chloroquine in many parts of the world, after the malaria parasite has become resistant to chloroquine – and to stamp certain autoimmune diseases such as rheumatoid arthritis and lupus, because of its ability to lower inflammation. The doctors began treating COVID-19 to make the drug after a small study in France suggested can help relieve inflammatory symptoms are some of the diseases of the respiratory tract and cause can compromise respiratory failure. The evidence for the effectiveness of the drug has not been found, although rigorous tests being randomly assign patients hydroxychloroquine or placebo is not yet complete (including one led by the National Institutes of Health). But there is growing evidence that the drug may not be as useful as doctors had hoped. In a study of more than 1,300 people in New York Presbyterian-Columbia University Irving Medical Center admitted, for example, hydroxychloroquine front desk people did not show lower rate of fans need or a lower risk during the study period that dying does not it is always the drug. to find studies from Europe and China similarly failed, that the drug did not show a benefit for patients. These studies have the US Food and Drug Administration (FDA) has led to alert health care providers that they should not prescribe hydroxychloroquine off-label if they are part of a research study or carefully monitored to COVID-19 patients in a hospital. In fact, as the evidence continues hydroxychloroquine may not bring much benefit, and instead may increase the risk of heart damage, he says Mehra doctors consider hydroxychloroquine with other medications can move as COVID-19 treatments, such as remdesivir investigated. Studies show that remdesivir for treating an antiviral for the treatment of every disease has not yet approved, people with COVID-19 can help you recover more quickly, and the drug has received FDA approval for hospital patients in emergency hospital. Unlike remdesivir hydroxychloroquine has not led to serious side effects previously associated with, and therefore more attractive option would be to treat 19 patients for COVID-doctors. “They may be more likely to try something where there is a better chance of a positive result,” says Mehra. “Why do you risk damage when there are so many Date consistent lack of benefit [View with hydroxychloroquine]?”
Related Post
COVID-19 is devastating even nursing homes. The administration Trump does not do much to stop it
At least 75,000 Americans in nursing homes and other structures have long-term care as soon as they died COVID-19 and the devastation is far from...
More than half of Americans fear that the pressure of the White House for a coronavirus vaccine will Rushed
For weeks, the US president Donald Trump has repeatedly forecasts, sometimes bordering on promises that a COVID-19 vaccine is imminent. "We remain on track to...
COVID-19 has killed nearly 200,000 Americans. How many more lives will be lost before the US makes it right?
Forty-five days before the announcement of the first suspected case of what the COVID-19 would be known, the global index of the Health Security has...
Zeneca has taken its COVID-19 vaccine trial after a break for the security check
This story has been updated to reflect AstraZeneca's investigational vaccine shot. AstraZeneca, the British pharmaceutical company behind one of the most promising candidates COVID-19 vaccine...
Nearly 2 million fewer US teens are vaping now than last year, the data show CDC
Nearly 2 million fewer US teens report on e-cigarettes in 2020 compared to 2019, according to new data from the US Centers for Disease Control...
The Great Race Vaccine: The unprecedented rush to immunize the world against COVID-19
The smarter enemies thrive on surprise attacks. Virus and crown in particular are well aware. remain hidden in animal hosts for decades, sometimes mutate constantly,...