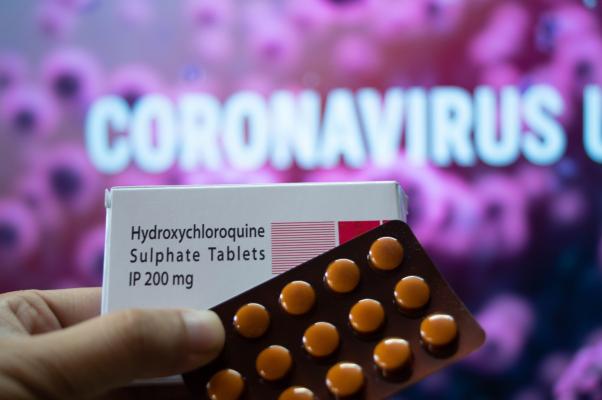
The Food and Drug Administration (FDA) announced today that the emergency use authorization for hydroxychloroquine and chloroquine removal of a lack of evidence, citing that these drugs are effective COVID-19. based, you can be found on thin evidence from a handful of relatively weak studies beginning pandemic doctors scrambling for a way to treat the disease widely prescribed hydroxychloroquine their sickest patients. However, recent research has increasingly clear that the drug is likely to be ineffective against the disease made, and that presents a risk of significant side effects, including heart problems. The chief scientist for the FDA, Denise M. Hinton, announced the decision in a letter to the Authority for Research and Advanced Biomedical Development (BARDA) on Monday. “Basic request to revoke Today on new information, including data from clinical BARDA results studies have come to believe that this drug may not be effective for the treatment of COVID-19,” he wrote, adding that “it is not reasonable” They believe that the benefits of the combination of drugs outweigh the possible side effects. Hydroxychloroquine is a slightly different version of chloroquine, a drug discovered to fight against malaria decades. Scientists have developed hydroxychloroquine as a substitute for the treatment of malaria, with fewer side effects; it is prescribed in the US today primarily to treat inflammation in autoimmune diseases such as rheumatoid arthritis and lupus together. While the United pandemic in March rose sharply, which has supported the minister Donald Trump hydroxychloroquine as a potential COVID-19 treatment, there is a possible appeal “game changer”. Trump encourages others to take the medication, and later claimed to know that a two-week treatment with hydroxychloroquine, in an attempt to have ward off disease. Medical experts have criticized President Trump a potentially dangerous drug for the promotion before his results were demonstrated, and warned of the possible risks to public self-Medica. The FDA said in a statement on Monday that the FDA a regular drug testing of emergency hospitalization in the context, and has been tested new information, including a large clinical study of hospitalized patients showed that the drug did not seem to convince people fast best to help, or reduce the risk of mortality. “While further studies continue to the potential benefits of these medications rate in the treatment or prevention of COVID-19, we realized that this was an emergency authorization is no longer appropriate. This action was taken after careful consideration by scientists in our Center for Drug Evaluation and Research, “Dr. Patrizia Cavazzoni, the acting director of the FDA’s Center for Drug Evaluation, said in the statement. -AFP Photo copyright by George Frey / Getty Images
Related Post
COVID-19 is devastating even nursing homes. The administration Trump does not do much to stop it
At least 75,000 Americans in nursing homes and other structures have long-term care as soon as they died COVID-19 and the devastation is far from...
More than half of Americans fear that the pressure of the White House for a coronavirus vaccine will Rushed
For weeks, the US president Donald Trump has repeatedly forecasts, sometimes bordering on promises that a COVID-19 vaccine is imminent. "We remain on track to...
COVID-19 has killed nearly 200,000 Americans. How many more lives will be lost before the US makes it right?
Forty-five days before the announcement of the first suspected case of what the COVID-19 would be known, the global index of the Health Security has...
Zeneca has taken its COVID-19 vaccine trial after a break for the security check
This story has been updated to reflect AstraZeneca's investigational vaccine shot. AstraZeneca, the British pharmaceutical company behind one of the most promising candidates COVID-19 vaccine...
Nearly 2 million fewer US teens are vaping now than last year, the data show CDC
Nearly 2 million fewer US teens report on e-cigarettes in 2020 compared to 2019, according to new data from the US Centers for Disease Control...
The Great Race Vaccine: The unprecedented rush to immunize the world against COVID-19
The smarter enemies thrive on surprise attacks. Virus and crown in particular are well aware. remain hidden in animal hosts for decades, sometimes mutate constantly,...