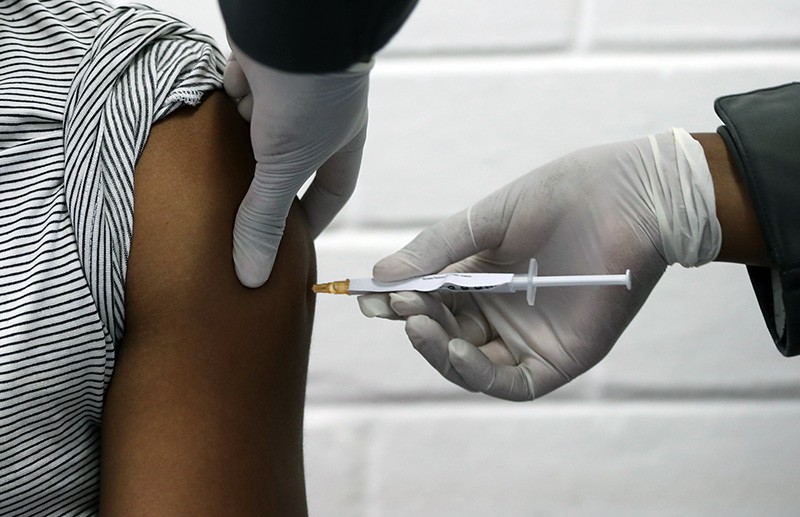
The foot race the first effective vaccine against COVID-19 includes terribly overcrowded, actually develop a field with 137 vaccine candidates in pre-clinical studies worldwide and another 23 under development. But a leader appeared in the research reports published in the Lancet produced today leads to a robust study by researchers at the University of Oxford in England promising. The study began in April, with a sample of 1,077 adults aged 18 to 55 a young enough age in contact band with COV2 SARS to tolerate the virus that COVID-19, caused with less risk of side effects than to see the elderly, vulnerable adults. The group was more or less divided into two halves, with 543 participants received experimental COVID-19 vaccine, and to obtain the other 534 as a portion of the control group, a vaccine against existing meningococcal vaccine. (The researchers did not chose an inert saline for the control group to be used as both side effects vaccines as tenderness, fever and fatigue can cause. Saline would not cause these symptoms and therefore would show which group was the group control and it was not.) the vaccine uses a chimpanzee adenovirus as a vector delivery harmless to humans. The virus is SARS-CoV-2 components of the crown wear spike proteins modified in theory the demand should trigger immune response in humans. What were the researchers were two types of immune response: humoral immunity and the production of antibodies to the virus system level; and cellular immunity, or by activating the immune system T cells infected with COVID-19 human cells attack viruses. You have a lot of what they sought. T cell responses to the vaccine was detected by day seven after the injection of the vaccine, reached the day 14 and remained at least increased until day 56. humoral antibodies reached day 28 and remained until day 56. This at least eight weeks of increased brand is to give a good measure of the effectiveness of the vaccine early, and the ability to at least the short-term immunity “see good immune responses in most,” Dr. Adrian Hill, professor of human genetics at ‘University of Oxford and one of the researchers in this particular study, told the Associated Press. “This vaccine What does particularly well trigger is two arms of the immune system.” It ‘really does not do more than that if the vaccine is administered. Of the 1,077 subjects, a handful were only 10 selected 28 for a second booster dose on days when they were still in the middle of their first dose of immunity. The small sample taken the risk of increased exposure limited to only a few topics. If antibodies from 37 of the subjects who received a single dose were tested against the virus in vitro or neutralized in one of them, or 62% tube 23, the fully until day 56. When the same virus in vitro study was conducted with 10 subjects who received a second dose, the neutralizing effect was a perfect 100%. The vaccine, for all its promise, is not yet ready for prime time. Phase two three tests to be performed also with many groups of larger samples with people who have or have COVID-19 already have. Moreover, it was a relatively young sample in use, in terms of safety, the vaccine should be tested in older people who, after all, require a much higher risk of complications and death as a result of COVID-19 and the group more effective vaccine. Some of these studies are already underway. A vaccine study involved a sample of 3,000 people started in Brazil in June. A similar process is started at the same time in South Africa. A large study of 30,000 people in the United States is to continue to come, and even planned to start this summer. But these early results are encouraging enough to help the pharmaceutical giant AstraZeneca decision vindicate Cambridge-based partner with Oxford. The company is engaged in mass production of 2 billion doses of a successful vaccine when completed. Oxford is not alone. Of the 23 vaccines in development, one more, Massachusetts ansässigem Moderna Inc., in collaboration with the National Institutes of Health, is running well ahead of the pack. In a Phase 1 trial week, 45 subjects the sample vaccine produces antibodies obtained, the SARS-CoV-2 in vitro neutralized effective as human antibodies that the disease had actually had. New York-based Pfizer will develop its own vaccine in a Phase I / II trial-safety testing, efficacy, side effects and dosage and ideal dose has shown similar results in promising level, with the snake already 90 million UK buy cans, once it has been approved. The Oxford study, the largest of its kind, brings us a significant step closer to the publication of a vaccination or an arsenal of vaccines-against-19 COVID pandemic. But we are not yet there. For the moment, the disease still raging and the world is still waiting.
Related Post
COVID-19 is devastating even nursing homes. The administration Trump does not do much to stop it
At least 75,000 Americans in nursing homes and other structures have long-term care as soon as they died COVID-19 and the devastation is far from...
More than half of Americans fear that the pressure of the White House for a coronavirus vaccine will Rushed
For weeks, the US president Donald Trump has repeatedly forecasts, sometimes bordering on promises that a COVID-19 vaccine is imminent. "We remain on track to...
COVID-19 has killed nearly 200,000 Americans. How many more lives will be lost before the US makes it right?
Forty-five days before the announcement of the first suspected case of what the COVID-19 would be known, the global index of the Health Security has...
Zeneca has taken its COVID-19 vaccine trial after a break for the security check
This story has been updated to reflect AstraZeneca's investigational vaccine shot. AstraZeneca, the British pharmaceutical company behind one of the most promising candidates COVID-19 vaccine...
Nearly 2 million fewer US teens are vaping now than last year, the data show CDC
Nearly 2 million fewer US teens report on e-cigarettes in 2020 compared to 2019, according to new data from the US Centers for Disease Control...
The Great Race Vaccine: The unprecedented rush to immunize the world against COVID-19
The smarter enemies thrive on surprise attacks. Virus and crown in particular are well aware. remain hidden in animal hosts for decades, sometimes mutate constantly,...