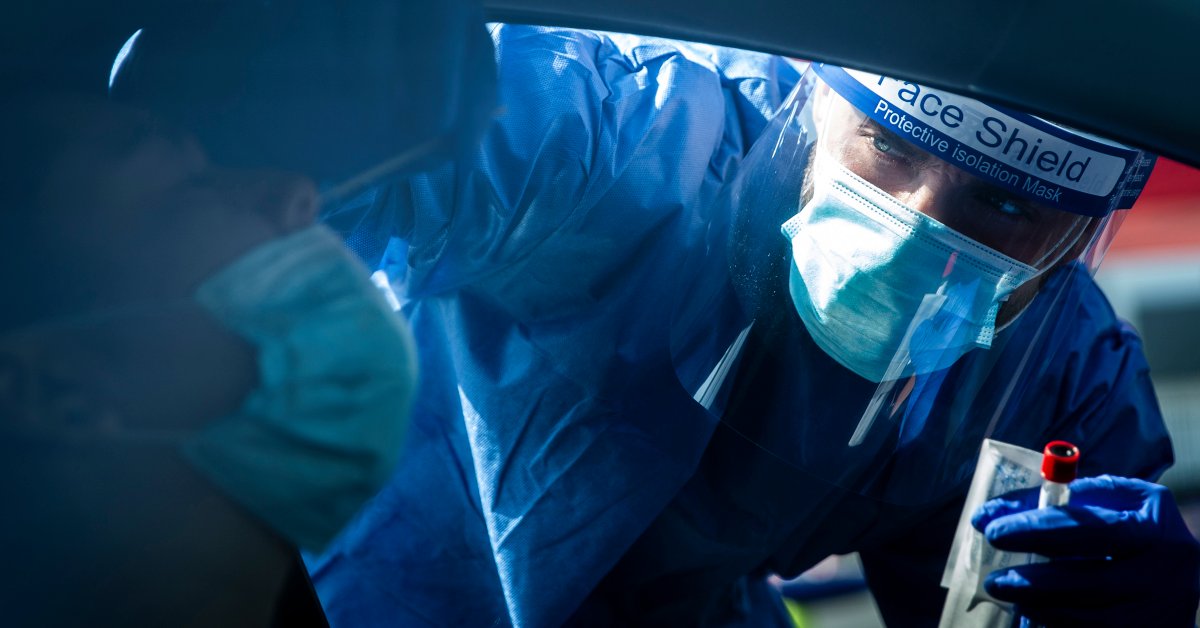
infrastructure to test America collapses. As rising crown cases across the country, the workshops can be cultured from samples before crippling shortage of key material and residues. In many states, it takes 10-15 days now to get the test results – to prevent these unnecessary tests as a means of rendering transfer and bring in pandemic control. For most people, the peak period of infectivity lasts about a week. And in the middle of this crash test, cities and municipalities still have to prepare this fall, millions of children go to school does not mean the opportunity to test them. At this critical moment in the struggle of our nation against COVID-19, testing time is radically rethink our approach. The way forward is not a perfect test, but offer quick results. In recent months, much it has focused the conversation to test for accuracy. Tests have been touted for their high sensitivity to correctly identify more than 98% of positive cases. The intuition is clear: we want a test, do not miss part of the positive cases and refer infected people in the world to spread the virus. This qPCR test has become the backbone of our test infrastructure, but their high cost and low rates have undermined the analysis attempts to put their high sensitivity for a good cause. CDC analysis suggests that we usually identify only about 1 in 10 cases of COVID-19, because it tries so few people. This means that the ability to identify positive cases to isolate from the point of view of public health and most importantly for Disease Control, are only about 10 percent. With a prize for the accuracy of the tests we fail a majority of people with COVID-19 and test made these delays, actually undermined our ability to identify specific cases which is the fundamental purpose for widespread testing. Imagine spitting on a special paper strip every morning and told two minutes later, if you were good for COVID-19 If everyone does this every day in the United States, there would be dramatically our transmission rates and lead to the fall of control in the event of a pandemic. Schools and businesses could be opened with the tranquility again that infectious persons had been identified and were at home. Michael Mina from the Harvard School of Public Health was an important member of this idea, and the idea has prompted a $1 trial, the government could mass produce and free to all. In fact, there are these technologies today. antigen tests are much cheaper and faster than qPCR and Quidel test received for their antigen test based on a piece of paper already FDA approval. Another $1 antigen test was taken in Senegal in use. Many other US companies to develop antigen test. Why is this a good market and rapid tests in order to be the basis of our national test strategy? The answer lies with the sensitivity of the test. antigen tests require higher virus as qPCR returned a positive result. E ‘became a significant push back from those who believe it would be irresponsible too far a test for the application, it may miss the many positive cases. But the frequency of testing and speed of outcome indicators that concern. QPCR currently lenses laboratory assays to a crawl. If each antigen test today requires only 50 percent of identifying positive there would still be 50 percent of all infections in the country being identified – five times more than 10 percent of the cases we are probably yet to be identified, because it tries so few people. Accuracy can be further increased by repeated trials and would be identified by the realization that rapid viral load results during the most infectious period, the cases means that identify the most important – in the peak period of infectivity less likely to lose. Even better, we identify these cases, while they are still infectious, rather than in 10 days if the virus could have been transmitted several times. Mina and colleagues have shown through modeling that this logic holds; The speed is to control how the sensitivity of the test in a much more of a pandemic. The evidence made clear that the time has come for a paradigm shift in testing. Our goal is to detect and prevent any cluster and every gust of COVID-19 cheap and quick antigen test can achieve this, although the sensitivity of the test does not match qPCR. It ‘s time for the federal government strong leadership to our resources to this new strategy to govern. The United States has the ability to test antigen paper for printing massive capacity and distribute it throughout the country. It has the ability to lead, antigen tests every day to tens of millions of Americans. If we do these things, we are able to move beyond the 10-day delay, compress the current outbreaks, and ensure that we can safely go to work, go shopping, and send our children to school. Picture copyright by Jenna Schoenefeld -The New York Times / Redux
Related Post
COVID-19 is devastating even nursing homes. The administration Trump does not do much to stop it
At least 75,000 Americans in nursing homes and other structures have long-term care as soon as they died COVID-19 and the devastation is far from...
More than half of Americans fear that the pressure of the White House for a coronavirus vaccine will Rushed
For weeks, the US president Donald Trump has repeatedly forecasts, sometimes bordering on promises that a COVID-19 vaccine is imminent. "We remain on track to...
COVID-19 has killed nearly 200,000 Americans. How many more lives will be lost before the US makes it right?
Forty-five days before the announcement of the first suspected case of what the COVID-19 would be known, the global index of the Health Security has...
Nearly 2 million fewer US teens are vaping now than last year, the data show CDC
Nearly 2 million fewer US teens report on e-cigarettes in 2020 compared to 2019, according to new data from the US Centers for Disease Control...
The Great Race Vaccine: The unprecedented rush to immunize the world against COVID-19
The smarter enemies thrive on surprise attacks. Virus and crown in particular are well aware. remain hidden in animal hosts for decades, sometimes mutate constantly,...
Zeneca has taken its COVID-19 vaccine trial after a break for the security check
This story has been updated to reflect AstraZeneca's investigational vaccine shot. AstraZeneca, the British pharmaceutical company behind one of the most promising candidates COVID-19 vaccine...