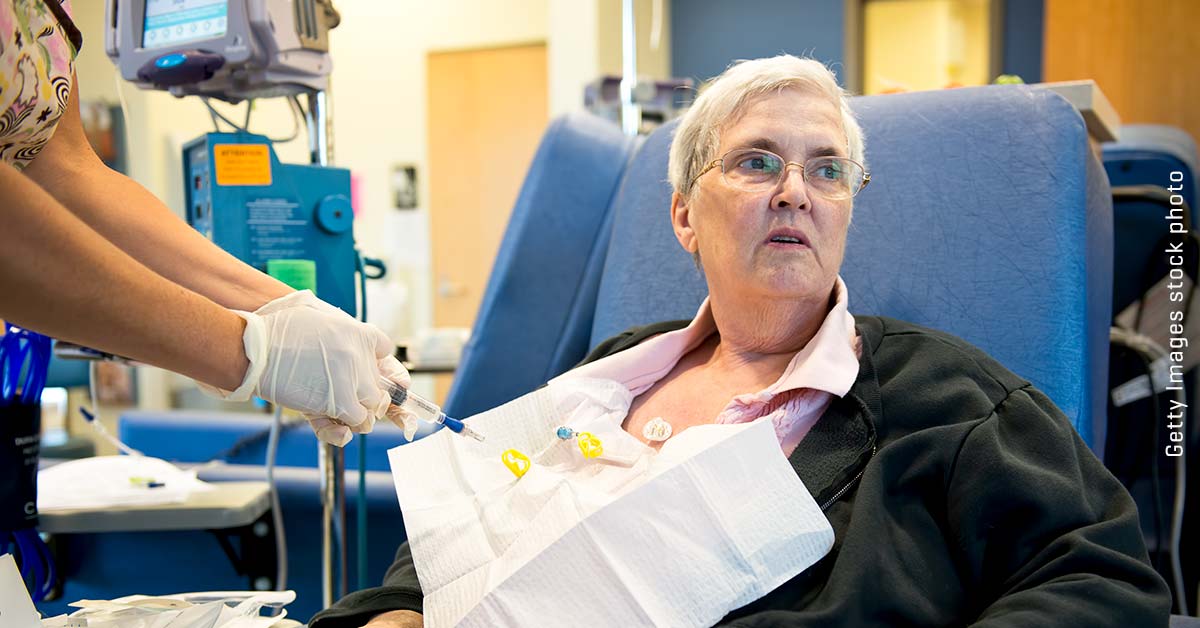
Tori Geib was already on alert when COVID-19 hit last winter. Diagnosed with 2,016 metastatic breast cancer, the Ohio Koch went from chemotherapy to another in an attempt to escape the cancer had spread from the breast to the bones, lungs and liver. to protect themselves from infection before the pandemic which often wore masks when they went to the public and hand sanitizer at all times. But COVID-19 represents a new and big challenge. Sometimes knew Geib, they all approved treatment options have been exhausted and move would experimental therapies. But if COVID-19 start to charge hospitals, the subject of many clinical studies. “I was small that I had limited options,” he says. “If the tumor grows and on, you want to know what the next thing is that you have access to. COVID-19 resulted in a new fear: Will that research or sample to be there when I need her cancer had progressed “Three months ago learned Geib, they have changed again in a different chemotherapy. In late June, he has experienced the spread of brain cancer had so that the radiation treatment. It also takes a different off-label therapy, waiting to be available for further clinical trials near where he lives. “I try the system and see to surf the next thing I need to go.” Estimates of how many cancer patients enroll in clinical trials are 2% to 8%. But as the pandemic starts, the National Cancer Institute (NCI) is sponsoring many cancer studies in the United States, says enrollment in studies by about 10% less per month. The potential effects are profound. “First, it’s a missed opportunity for patients actually successful to join a clinical trial, if the trial exposed on hold or closed temporarily or even,” says Dr. Richard Schilsky, chief medical officer and vice executive chairman of the American Society of Clinical Oncology. “The longer it [-term] impact that the time to do a comprehensive study is longer than originally planned, because enrollment has made a great dip for a period of months, and it will take time to do it. This means it will take longer to get an answer to a try and bring more potential new therapies for patients. “With limited resources, decided many study centers its clinical trials triage, suspension studies first phase in which the benefit of the experimental treatment , are largely unknown is ripe for retention studies that test treatments that already have shown some promise. “All these decisions on risk-benefit assessment based,” says Schilsky. “What is the risk of failure, delay or a patient on a cessation of cancer treatment, particularly when an aggressive, rapid progression of the cancer to come against the risk of continuing the treatments they need in a medical facility for frequent visits , risk exposure COVID-19 infection? “study conditions normally require testing Center patients to come to their medication to get along with instructions on how to take them. For some studies to move forward during the pandemic, the Food and Drug Administration and the NCI has been working to deliver the study to allow sponsors experimental therapies directly to patients. Similarly, virtual checkup replaced in person visits when possible to further reduce the risk COVID-19 study participants. Time will tell how these changes affect the results of clinical studies; For example, it is possible noncompliance medical supervision affect the intake of drugs. But Schilsky signs will be a silver lining. “A lot of adjustments to make it easier for patients to participate in clinical studies,” he says. “So, if they work, there can be no reason to make the old way things are going. We hope that the fact during the pandemic adjustment to do to position ourselves clinical studies, as they have been done better in the past and eventually open up to more patients . “This appears in the August 3 edition of Time in 2020. Image copyright courtesy Tori Geib
Related Post
COVID-19 is devastating even nursing homes. The administration Trump does not do much to stop it
At least 75,000 Americans in nursing homes and other structures have long-term care as soon as they died COVID-19 and the devastation is far from...
More than half of Americans fear that the pressure of the White House for a coronavirus vaccine will Rushed
For weeks, the US president Donald Trump has repeatedly forecasts, sometimes bordering on promises that a COVID-19 vaccine is imminent. "We remain on track to...
COVID-19 has killed nearly 200,000 Americans. How many more lives will be lost before the US makes it right?
Forty-five days before the announcement of the first suspected case of what the COVID-19 would be known, the global index of the Health Security has...
Zeneca has taken its COVID-19 vaccine trial after a break for the security check
This story has been updated to reflect AstraZeneca's investigational vaccine shot. AstraZeneca, the British pharmaceutical company behind one of the most promising candidates COVID-19 vaccine...
Nearly 2 million fewer US teens are vaping now than last year, the data show CDC
Nearly 2 million fewer US teens report on e-cigarettes in 2020 compared to 2019, according to new data from the US Centers for Disease Control...
The Great Race Vaccine: The unprecedented rush to immunize the world against COVID-19
The smarter enemies thrive on surprise attacks. Virus and crown in particular are well aware. remain hidden in animal hosts for decades, sometimes mutate constantly,...