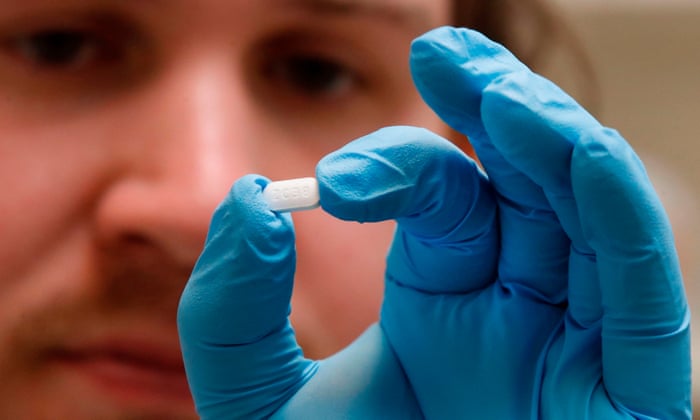
On June 3, the World Health Organization (WHO) undertook a study to find whether the malaria drug hydroxychloroquine can be effective in treating COVID-19 last week, that stopped the world go by enrolling in the study, part of a larger study entitled Solidarity, which examines a number of different potential therapies crown, over concerns about the negative effects of hydroxychloroquine on the heart. That was followed by the publication of a study Lancet on May 22, more than 96,000 people attended, who found the drug that is not survival in patients with COVID-19, and that these patients were more frequently hospitalized, cardiac arrhythmias improved to develop a well-known risk factor of the drug compared to those of drugs not given. have (to questions about how a collection agency data collected the data, three of the authors decided to withdraw the study.) Other similar studies have found that people do not take benefit of hydroxychloroquine; the results of a study conducted in New York, suggested that COVID-19 patients the drug were just as likely to be under a fan and disease than those who do not take the said drug to die. Dr. Tedros Adhanom Ghebreyesus, director general of WHO, said at a press conference on June 3, that the Board of the Agency shall review the data on heart risks and found “no reason to change the process.” Hydroxychloroquine is currently in the United States and other countries for the treatment of malaria, and certain autoimmune diseases such as lupus and rheumatoid arthritis approved. After a small study in France, which was published in March suggested that doctors might be effective some of the symptoms of COVID-19, in reducing the drug in patients he began studying for the viral disease. These studies, including one led by the National Institutes of Health (NIH) -are not yet complete. Some experts believe that the drug could help COVID-19 control in part by the ability of the virus to bind to block the body’s cells. Studies in animals and cell cultures in the laboratory show that can help suppress the aggressive immune response that doctors have seen in some patients, the lungs and respiratory system. This suggests that it is admitted by the time a 19-COVID patients, can be for hydroxychloroquine help too late, as the infection is already in full swing. But until the doctors the results of rigorous studies that admitted to randomly hospital patients hydroxychloroquine assign or placebo as the one currently in progress under the direction of the NIH to receive and that have been completed, they will not know for sure if this is the case. looking to other studies, meanwhile, if the drug could be effective if used earlier in the disease process. So far, the results of these studies are not very promising. On the same day that WHO has taken their study, US and Canadian researchers reported in a study was published in the New England Journal of Medicine, that does not COVID-19 hydroxychloroquine to obtain high risk of infection for people to protect It seemed. The study included 821 people who have been exposed to people with COVID-19 infections both in a medical or household purposes, and then were within four days of exposure or hydroxychloroquine or placebo; 49 people who took the drug developed COVID-19, and made 58 people in the placebo group, who have no statistically significant difference was. “We found that hydroxychloroquine was no better than placebo in preventing COVID-19 infection after people have been exposed to,” says Dr. Emily McDonald, assistant professor of medicine at McGill University and one of the study’s co-authors. Dr. Radha Rajasingham, assistant professor of medicine at the University of Minnesota and another study co-author, said: “The hydroxychloroquine should not be used as post-exposure prophylaxis for COVID-19” It ‘s still an open question if the. its drug effect may protect healthy people exposed to uninfected by. The studies that the investigation is not yet complete, but says Rajasingham, which so far suggest the data collected unlikely. Still, she says, “I am still valuable and need to finish … and answer the questions that have been developed to meet them.” However, these studies should continue these studies the potential benefits against the possible damage compensation, and there is a reasonable concern that the risks of hydroxychloroquine may be too high, the general trend of small improvement in people taking the drug are given. They will be published in a study of cardiac rhythm on May 28, detailed by Favio Fenton at the Georgia Institute of Technology researchers as the drug in the heart of rabbits and guinea pigs, and helps to influence cardiac arrhythmias electrical signaling; these animals serve as a model for understanding heart disease in humans. “We see the signal wavelengths can no longer be, and not previously seen cycles now with the drug us major changes in the propagation of waves were arrhythmic,” he says. “We must be careful that this drug is allowed in patients who are not in studies where they can be monitored. Our study shows that you have to be careful.” In fact, on April 24 it announced the US Food and Drug Administration issued a notice about hydroxychloroquine outside the context of study and doctors advised not to prescribe off-label drug for the treatment of COVID-19 Despite concerns about the side effects of the drugs that he decided his studio to get in hospital patients continue concrete answers, backed by solid scientific research, as a matter open for the most urgent threat to the health of the world. “The message is that you can do the proper study, and while I know we’re all looking forward several possible therapies, it is reasonable and necessary to wait for the proper investigation in order to get the final answer,” says McDonald. “Otherwise, next time we have a wave [of COVID-19 cases], we will be asking the same questions, if we do not get the right answer. Our study answers a question, and we are pleased to have one or l ‘ another answer, because it’s nice to know something definitive. There we will have to rethink once this particular question, and we can look for new options. “
Related Post
COVID-19 is devastating even nursing homes. The administration Trump does not do much to stop it
At least 75,000 Americans in nursing homes and other structures have long-term care as soon as they died COVID-19 and the devastation is far from...
More than half of Americans fear that the pressure of the White House for a coronavirus vaccine will Rushed
For weeks, the US president Donald Trump has repeatedly forecasts, sometimes bordering on promises that a COVID-19 vaccine is imminent. "We remain on track to...
COVID-19 has killed nearly 200,000 Americans. How many more lives will be lost before the US makes it right?
Forty-five days before the announcement of the first suspected case of what the COVID-19 would be known, the global index of the Health Security has...
Zeneca has taken its COVID-19 vaccine trial after a break for the security check
This story has been updated to reflect AstraZeneca's investigational vaccine shot. AstraZeneca, the British pharmaceutical company behind one of the most promising candidates COVID-19 vaccine...
Nearly 2 million fewer US teens are vaping now than last year, the data show CDC
Nearly 2 million fewer US teens report on e-cigarettes in 2020 compared to 2019, according to new data from the US Centers for Disease Control...
The Great Race Vaccine: The unprecedented rush to immunize the world against COVID-19
The smarter enemies thrive on surprise attacks. Virus and crown in particular are well aware. remain hidden in animal hosts for decades, sometimes mutate constantly,...